Functional Description
Measuring principle
In industry and laboratories the measurement of the oxygen concentration in gases is often required. Mostly, it is measured in gases which have a remarkable temperature independent oxygen concentration.
|
|
The NERNST-equation is used as a basis for determining the oxygen concentration in gases with the oxygen monitor OTM.
|
NERNST-Equation
|
|
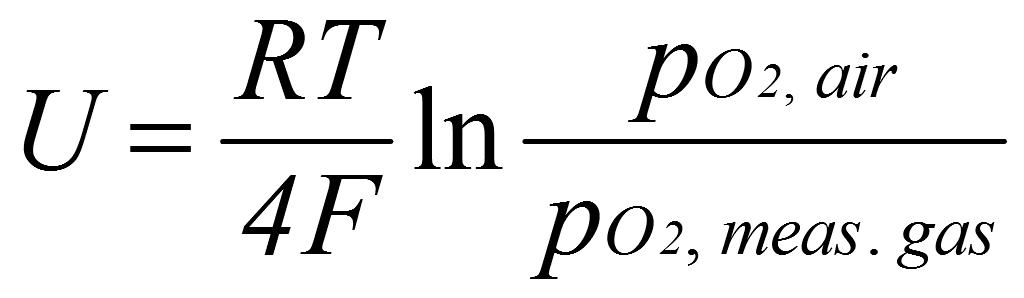
|
(I)
|
|
U – cell voltage in V
R – molar gas constant, R = 8,314 J/(mol · K)
T – measuring temperature in K
F – Faraday-constant, F = 9,648 · 104 C/mol
pO2,air – partial pressure of the oxygen at the reference electrode
in dry air in Pa
pO2,meas.gas – partial pressure of the oxygen at the measuring electrode
in the measured gas in Pa.
The sensor of the OTM is based on the conductivity of oxide ions in a special ceramic substance (zirconium dioxide) with stabilising additives. The conductivity of these oxide ions increases exponentially with the temperature and it is sufficiently high for temperatures above 600°C.
The gas to be measured passes through the ceramic oxide ion conductor which is a gas-tight pipe. The ceramic pipe is situated axially symmetric in a thermally well-insulated heater. The electrodes of the galvanic sensor are made from platinum. The electrode on the outside of the pipe, surrounded by dry air, is used as a reference electrode with a constant, known electrode potential.
Based on the assumption that the total pressures of the gases are almost the same at both electrodes (in this case the volume concentrations may be used in the calculation instead of the partial pressures) and replacing the parameters by numbers in equation (I) the following equation is valid:
|
Equation
for Oxygen Concentration
|
φO2= 20,64 · e (-46,42 · )
|
(II)
|
φO2 – oxygen concentration in the measured gas in vol.-%
U – potential difference in mV
T – measuring temperature in K
20,64 – oxygen concentration in air with a relative humidity of 50% in vol.-%.
Measuring conditions
General recommendations
The oxygen may be in free or bound form inside the measuring gas.
Thereby, the following dependencies are valid:
– for free oxygen
– for bound oxygen
The equation (II) for calculating the oxygen concentration is valid for measured gases with free oxygen as well as for reducing gas mixtures in which oxygen only exists in bound form (e.g. in H2/H2O- or CO/CO2-mixtures).
In reducing gas mixtures, the oxygen partial pressure is inversely proportional to the temperature. For converting the measured value at the measuring temperature into other temperatures special thermodynamic equations are required.
|
Reducing Gas Mixtures
|
|
|
Gas flow quantity
For exact measurements a gas flow between 5 and 10 l/h of the measured gases must be assured. If the flow is higher or lower than indicated, an additional measuring error arises.
|
|
At lower quantities, the measurement results become distorted by contamination effects of the gas pipes (leakages, permeability, desertions). If the gas flow trough the sensor too much, asymmetric cooling of the sensor electrode may cause additional measuring errors.
The gas flow is monitored by electronic. The internal pump if it is available is controlled by these signal. At use of a removed measuring cell (OTMS M1) the gas flow has to be adjusted with a needle valve according to the flow meter.
The OTM delivers an error message at transgression or under run of the mentioned limiting values. But the measurement are continued.
Accuracy of the measurement
The manufacturer guarantees a measuring error of less than 3% (relative error) only with measurements of oxygen concentrations within a range of 2 · 105 ... 10 ppm*)*. For measurements of oxygen concentrations of 10 ... 10-3 ppm the relative error is less than 5% if the gas inlet pipe has no leakages or permeability’s.
For measurements of oxygen concentrations less than 10 ppm, the following aspects must be taken into account during evaluation:
• composition of the measured gas
• specific characteristics of the production process (e.g. material used);
• temperature of the measured gas.
To reduce the measuring error at low oxygen concentrations, the following advice must be considered:
|
|
• The measuring gas must be taken from a location where the measuring gas has a homogeneous concentration of its substances.
• The pipe from the measuring point to the OTM must be as short as possible in order to avoid a change in the chemical balance in the pipe.
• All gas inlet and outlet pipes must be free of leakages.
• For measurements of oxygen concentrations of less than
1000 ppm, the use of steel pipes is necessary.
|