paramagnetic oxygen sensor (para O2
description
Oxygen distinguishes itself from other gases by its paramagnetic behaviour. In addition to oxygen, only NO, N02, and Cl02 are paramagnetic, too, while all other gases are slightly diamagnetic.
The paramagnetism of the measurement gas is inversely proportional to the temperature, where as the diamagnetism is independent of the temperature. This property which is utilized with the present measuring principle effects that the oxygen-containing cold gas is attracted more strongly by a magnetic field than when being in its warmed up condition.
Mode of operation
Oxygen distinguishes itself from other gases by its paramagnetic behaviour. In addition to oxygen, only NO, N02, and Cl02 are paramagnetic, too, while all other gases are slightly diamagnetic.
The paramagnetism of the measurement gas is inversely proportional to the temperature, where as the diamagnetism is independent of the temperature. This property which is utilized with the present measuring principle effects that the oxygen-containing cold gas is attracted more strongly by a magnetic field than when being in its warmed up condition.
Figure 1 shows the measurement principle of the thermomagnetic detecting element.
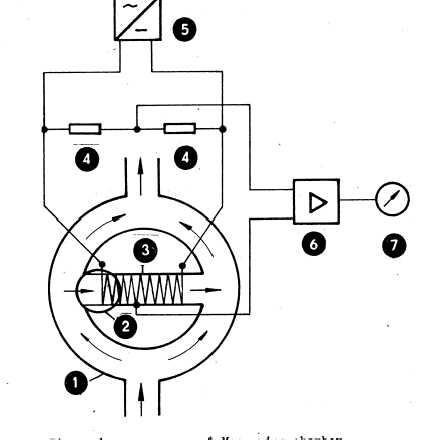
Figure 1
1 Measuring chamber
2 Magnetic field
3 Glass tube with platinum
filament winding
4 Fixed resistor
5 Constant voltage
6 Standard signal amplifier
7 Digital display
Within the oxygen concentration measuring instrument PERMOLYT 3, the gas to be measured flows through the annular measuring chamber 1 which is interconnected by a horizontal thin walled glass
tube (3). The glass tube is provided with two parallally arranged platinum filament windings which, together with two fixed resistors, are constituent parts of a Wheatstone bridge. The left platinum filament winding is located in the magnetic field (2) of a permanent magnet.
The two active measuring-bridge resistors are heated up in a defined manner by the constant-voltage source. If oxygen containing gas flows through the measuring chamber, the paramagnetic- oxygen molecules are drawn into the magnetic field of a strong permanent magnet. As the paramagnetism decreases when the temperature increases, the movement velocity of the oxygen molecules is lowered according to the warming-up increasing toward the right side.
Within the glass tube, a cross-flow from the left-hand side to the right-hand side is formed which is proportional to the oxygen concentration.
The flow within the glass tube causes a temperature change of the platinum filament winding which due to the resulting resistance change varies the equilibrium of the measuring bridge. The diagonal voltage caused by the unbalance of the measuring bridge is converted by the measuring amplifier (6) into the standard signal visible on a digital display (7).
In case of devices with suppressed zero range, the measuring chamber (1) is turned so that the glass tube (3) deviates from the horizontal nominal position. Thereby, a convection flow is formed which effects suppression of the measuring initial-value range if it is inverse to the measuring effect.
The inclination-independent special design of the detecting element contains two glass tubes (1) and thus is provided with four active measuring bridge resistors. Deviating from Figure 1, the two glass tubes are parallelly arranged within the measuring chamber, with one glass tube being exposed to the
magnetic field at the left-hand side and the other one the right-hand side. Due to this symmetric construction, convection flow is compensated which occurs during rotation of the measuring chamber. Formation of the measured .value and further signal treatment are carried out as described above.